In the field of inorganic nanoparticles (NPs) for biomedical applications, most future researches aim at developing multifunctional theranostic NPs (i.e.) that include simultaneously therapeutic and diagnostic/imaging functions allowing to envision image-guided therapy. In that context, we focused our research in the engineering of mesoporous silica (MS) based nanotheranostics designed for drug delivery, hyperthermia and imaging for cancer treatment. There are still very scarce silica based systems investigated in the clinical phases able to achieve such tasks which remain a key challenge in the nanomedecine field.
In our team, we developed different strategies these last years to adress these questions:
- Silica-templated protein nanocarriers for drug delivery
- Stellate large pore mesoporous silica nanocarriers for in vivo bio-imaging, biodepollution and protein release applications
- Activable iron oxide core@ MS shell for MRI, magnetic hyperthermia, photothermia
- NIR light activable carbon core@MS shell for photothermia and drug delivery
4-1. Silica-templated protein NPs for drug delivery to cancer cells
A key originality of this study is the protein assembly method itself. We use a pioneering method for non-covalent macromolecular assembly based on isobutyramide (IBAM) groups. Such assembly approach allows the formation of original self-supported micron and submicron size particles made of proteins and also other biomacromolecules obtained by a templating method where silica (plain or porous) is used as a sacrificial template. The macromolecules are held together via physical bridging with IBAM-graft that are hypothesized to originate from strong hydrogen-bonds.
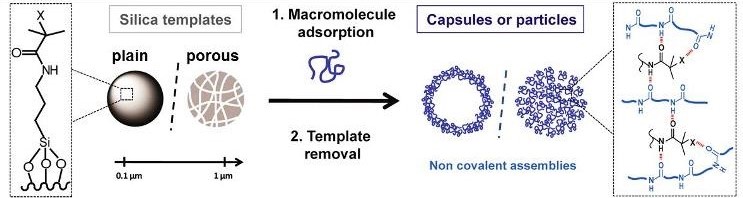
Scheme illustrating the formation of silica templated-assembly of submicron capsules and particles.
One challenge was to move from the usual micron-scale to the sub-micron-scale. Human serum albumin (HSA), in addition to its intrinsic properties of biocompatibility, biodegradability and non-immunogenicity, is particularly promising to enhance NPs circulation time in the blood. Here, we achieved the formation of homogenous HSA nanocapsules (30-60 nm size) loaded with and antitumor drug, doxorubicine (DOX) having a high drug payload (close 80% wt./protein NPs).
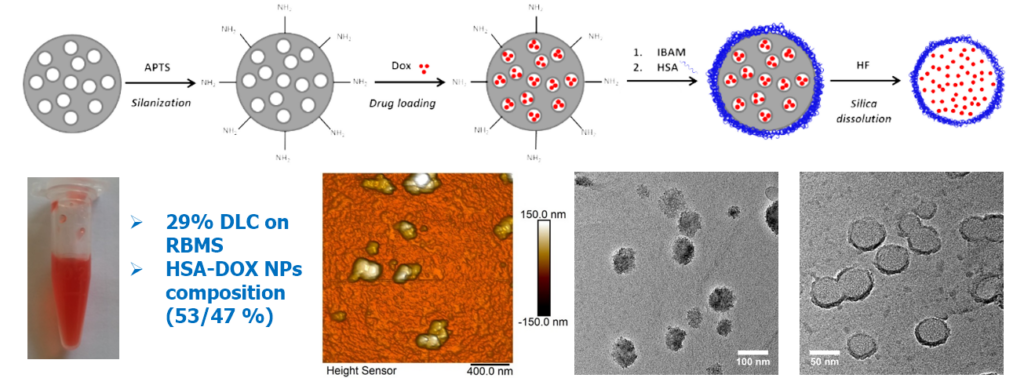
Design of templated protein capsules as drug delivery systems. BBA – Gen. Subj. 2019, 1863, 332-341.
The drug delivery from such nanocapsules was evaluated in multicellular tumor spheroid (MCTS) models made from Huh-7 (hepatocarcinoma, liver cancer) cells in coll. with Prof. F. Meyer (INSERM U1121). Results shown that such MCTS mimicking metastasis, exhibited a strong inhibition that can be related to a massive cancer cell death due to the antitumor drug release.
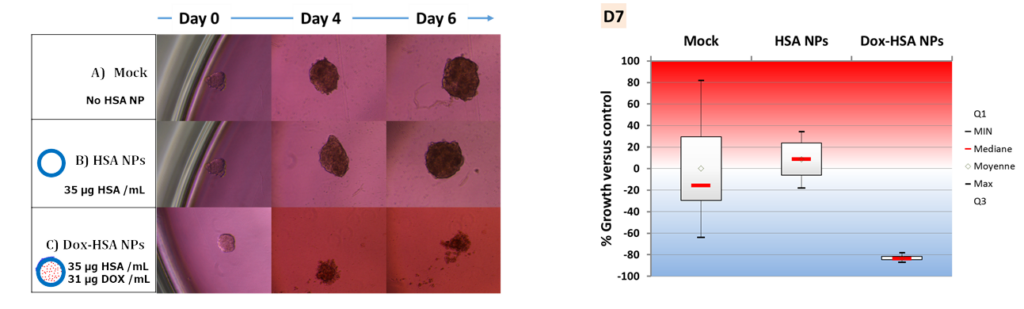
Effect of the drug-loaded protein nanocapsules on hepatocarcinoma spheroid cancer cell models. BBA – Gen. Subj. 2019, 1863, 332-341.
4-2 Stellate large pore mesoporous silica for nanomedecine
– Fluorescent stellate silica probes for in vivo bioimaging
In this work, we reported on an original strategy for the hierarchical assembly of novel biocompatible luminescent nanoplatforms. They consist in stellate large pore STMS grafted with InP/ZnS quantum dots (QDs) with a very high efficiency, which are then protected by a tight polysaccharide capping. These luminescent nanoplatforms were investigated in vivo in coll. with Dr S. Harlepp and Dr J. Goetz (INSERM U1109). As visualized by in vivo confocal macroscopy imaging within zebrafish (ZF) translucent embryos, these nanoplatforms are shown to rapidly extravasate from blood circulation to settle in neighboring tissues, ensuring a remanent fluorescent labelling of ZF tissues in vivo.
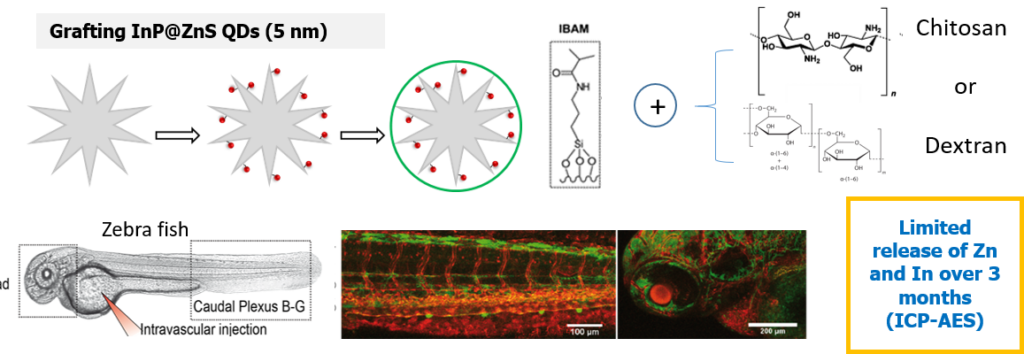
– Biodepollution: metal capture by stellate silica
The challenge of this work was the design of a new functionalized large pore silica efficient for the specific capture of iron from a biological environment. Naturally, living organisms tightly control iron homeostatic level but its overload can occur in some genetic dysregulations such as hemochromatosis. Herein, STMS NPs were covalently linked to the highly specific and natural iron chelating agent desferrioxamine B, denoted DFoB. DFoB is already used in its free form for iron capture in body, but presents limitations such as a rapid bioelimination. Here implementation for the first time of its chelating property when it is covalently immobilized on STMS brings advantages such as increasing circulation time, or incorporating in the future other functions such as a magnetic core for magnetic recycling. Regarding the selectivity of the removal process, the NPs were found efficient in Barth’s buffer mimicking the brain ionic solution composition. The objectives of this work were first the step by step build-up and characterization of the DFoB functionalized nanoplatforms and the evaluation of its chelating efficiency and selectivity towards iron capture. (Coll.Dr M. El-Habiri, LIMA, Strasbourg and C. Bertagnolli and A. Boos, IPHC, DSA, Strasbourg)
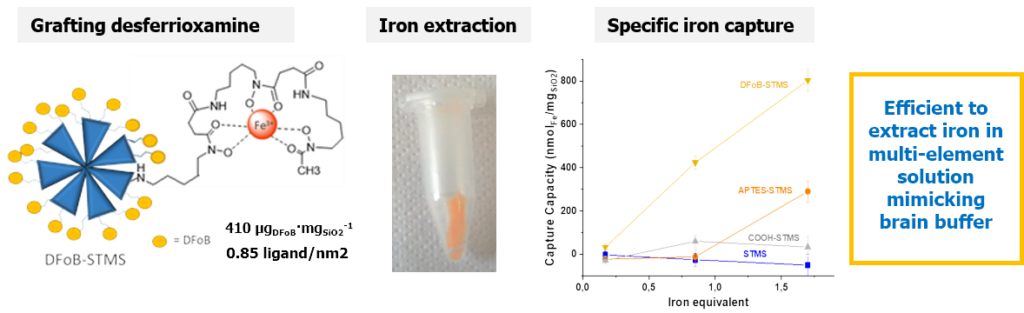
Design of STMS grafted with highly chelating ligand desferrioxamine and its specific iron capture. J. Colloid Interface Sci. 2020, 579, 140-151.
– Large pore silica for protein loading and release
Proteins have been studied for decades as therapeutic agents due to several advantages over synthesized molecules. However, among their disadvantages, proteins suffer from a short half-life and a conformational fragility, making them delicate for a direct use in therapy. Thus, there is a need to develop protein delivery systems (PDS) able to load and deliver them at the injured site. Among PDS, mesoporous silica NPs (MSNs) are promising, especially the large pore STMS. In this work, we investigated the original intermolecular glue strategy at STMS NP interface, based on isobutyramide (IBAM) binders, to evaluate its potential for protein sustained release in biological aqueous buffer. Herein, these questions were investigated in depth by using four proteins: human serum albumin (HSA), horseradish peroxidase (HRP), immunoglobulin G (IgG) and poly-L-lysine (PLL). A first aim of this work was to quantify the loading capacity of these proteins on STMS@IBAM which was done using three different detections techniques to determine with accuracy and precision the loading capacity in our NPs as a function of their feed weight ratio (FWR). Secondly, after a preliminary study which allowed to set the physicochemical properties and the chemical engineering conditions of the protein-coated NPs, the ability of the NPs for sustained protein release in HEPES buffer (pH 7.4, 50 mmol.L-1) was evaluated and demonstrated.
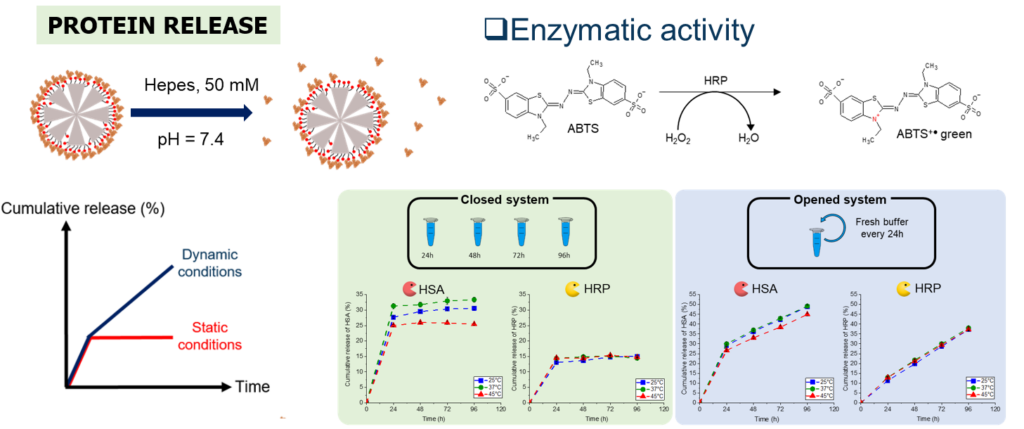
Protein sustained release from IBAM-modified STMS NPs and validation of enzymatic activity. Int. J. Pharm. 2022, 100130.
4-3 Theranostic magnetic core-mesoporous silica shell
In this part, we developed magnetic core-MS shell having various core size or pore morphology (small SPMS vs large STMS pores) for investigating possibilities to combine MRI, magnetic hyperthermia and drug delivery in cancer cells.
– Drug delivery in cancer cells and MRI
Herein, doxorubicin-loaded IO@SPMS capped with serum albumin were designed to achieve drug release to hepatocarcinoma (Huh-7) spheroid cells (coll. with Prof. F. Meyer INSERM U1121) and T2-weighted MRI.
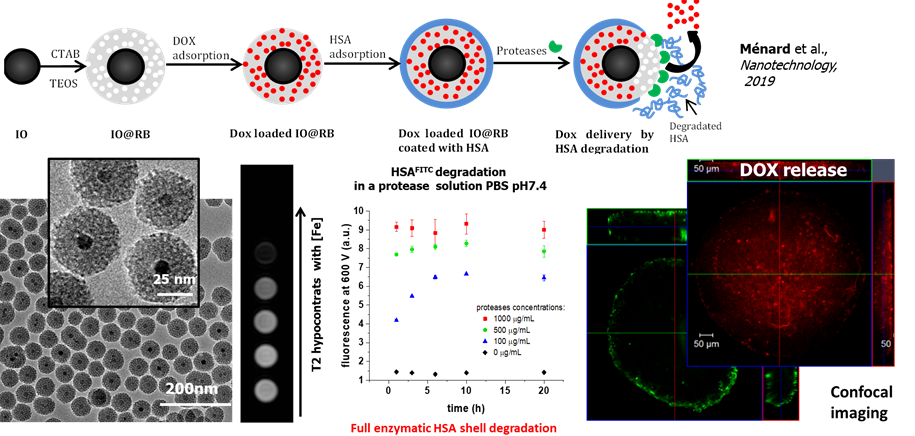
Magnetic core shell having small pores for MRI and drug release. Nanotechnology 2019, 30 (17), 174001
– IO@STMS NPs endowed with magnetic hyperthermia and MRI
In a second work, using a bigger core suitable for magnetic hyperthermia applications covered with larger pore silica shell, we grafted QDs to the large pore and we capped the surface with HSA. This resulted in a multifunctional magnetic core-shell nanocomposite (100 nm size) displaying bimodal imaging (MRI and fluorescence) coupled with magnetic hyperthermia. The NPs under AMF allowed to kill Hela cancer cells in combination of free antitumor drugs using only a low amount of NPs. (Coll. Dr M. Tasso, CONICET, Univ La Plata, Argentina)
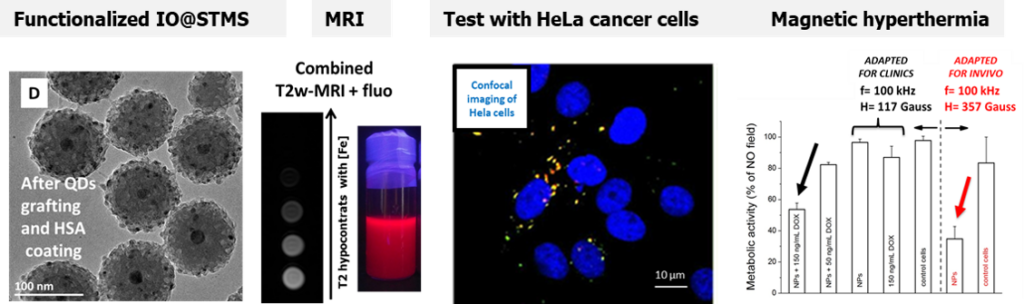
Fluorescent and Magnetic Stellate Mesoporous Silica for Bimodal Imaging and Magneic Hyperthermia. Appl. Mater. Today, 2019,16, 301-314
– IO@STMS NPs endowed with NIR light induced photothermia and drug delivery
In this work, we addressed for the first time the use of IO@STMS for combined photothermal and drug release properties. ST morphology has been chosen as their large pores promote the drug loading and improve heat dissipation from the central core to the surrounding medium. First, the photothermal properties of these core-shell NPs upon NIR light excitation were obtained by tracing temperature profiles and evaluating their specific absorption rates (SAR) as a function of the laser power and the NPs’ concentration. Secondly, the effect of key parameters, such as the pH of the drug impregnation aqueous solution, the surface chemistry or the presence of an iron oxide core (STMS vs. IO@STMS) on the drug loading capacity were evaluated.
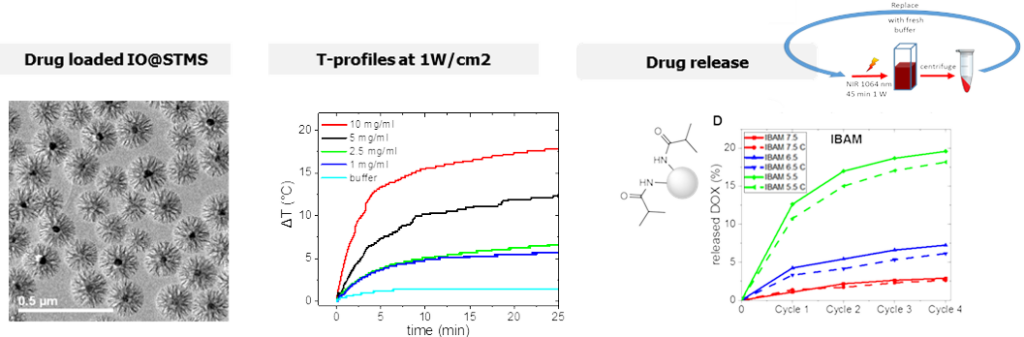
IO@STMS NPs used for combined NIR-light induced photothermia and drug release properties. Colloids Surf. A: Physicochem. Eng. Asp. 2022 640, 138407.
4-4 Carbon@silica core shell for photothermia and drug delivery
In this work, the design of a new class of carbon-based MS materials based on carbon nanotubes (CNTs) and few layer graphene (FLGs) as core systems was achieved for NIR-light photothermia combined with drug delivery applications. MS coatings strategies were applied around two C-based materials with a great control over the shell thickness. Drug loading onto these C@MS materials was investigated with varying parameters (drug concentration, surface modification) and showed a great capacity of drug payload (≥ 100%wt).
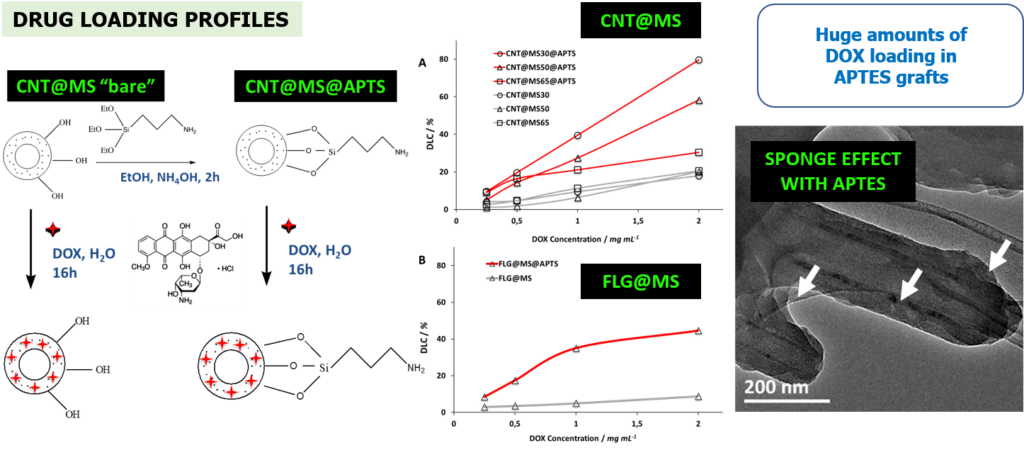
Engineering of mesoporous silica coated carbon materials optimized for an ultra-high doxorubicin payload. Adv. Funct. Mater. 2018, 28, 1706996.
Regarding drug release, we showed that a fraction of drugs could be released with different local or remote stimuli applied on such composites: under low acidic conditions (pH ca. 4), or by applications of a NIR light. At least, such drug loaded CNTs@MS NPs were evaluated in contact to D2A1 cell line (mouse breast carcinoma tumor). Results indicate that such NPs are able to kill cancer cells by photothermic effect under NIR light and ensure drug release activated by photothermal effect.
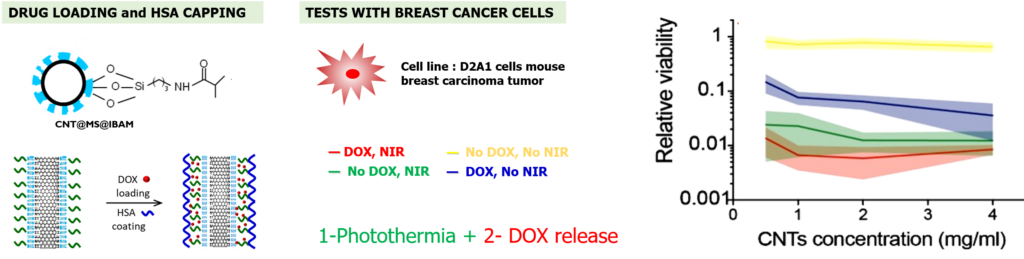
Photothermia and drug release activated by NIR light tested on breast cancer cells.
Mater Today Chem 2020, 17, 100308
