The design of smart responsive nanocomposite scaffolds emerged these last years as a powerful strategy for applications in nanomedicine. For instance, in some antitumoral treatments, key advantages are highlighted in the use of implantable or injectable matrixes alternatively to blood circulating nanomaterials. Indeed, by achieving a sustained and even controlled delivery of therapeutics to a specific diseased tissue, these implants may ensure a local delivery of antitumor drugs to fight cancer, with an optimized dose and minimum loss of drugs administered avoiding side effects.
In this Topic, our aim is to design such types of responsive nanomposite scaffolds based on our expertise on polymer self-assembly and on the chemical synthesis of original nanomaterials as described in the other Topics (ex: large pore STMS, iron oxide core@ STMS, carbon nanotubes@MS nanocomposites etc…).
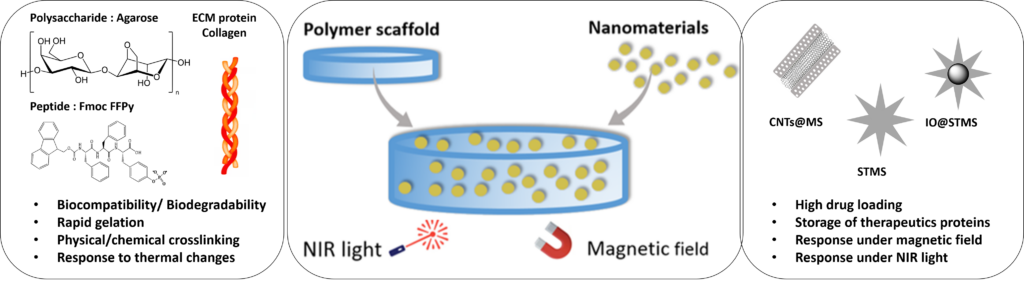
Building blocks used to design our nanocomposite hydrogels and the external stimuli of such new materials
The chemical design is very important. The challenge is not only in the formulation of a new generation of hydrogel nanocomposites with appealing properties, but it is also the implementation of innovative chemical approaches to architecture such nanocomposite hydrogels. This may be possible by creating smart connectivity between the nanomaterials and the polymer during gelation and by triggering specific localized interactions or biochemical reactions initiating hydrogelation at the surface of the nanomaterial itself.
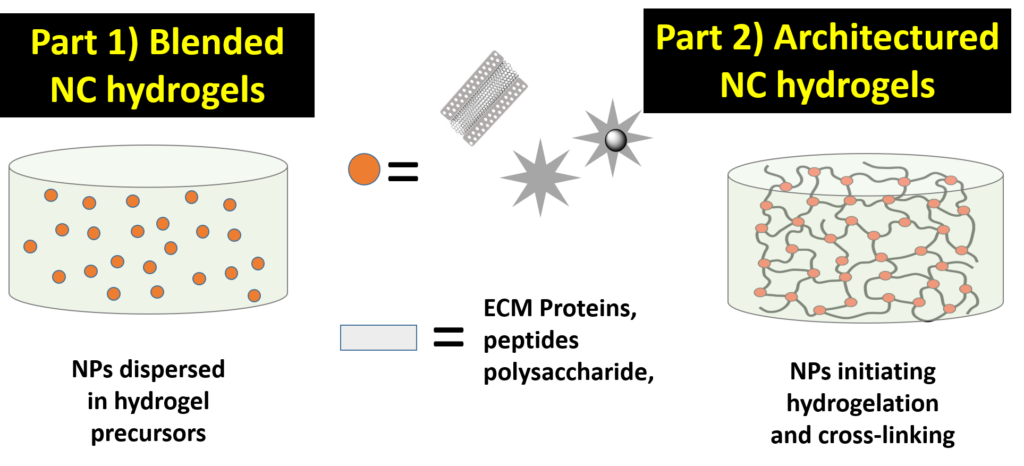
Illustration of the ways used to design our nanocomposite hydrogels which will described in two parts in this Topic. Way I. Mixed NC hydrogels. Way II Architectured NC hydrogels.
6-1 Blended functional nanocomposite hydrogels
– NIR-light induced drug release from nanocomposite hydrogel to cancer cells
Previously, anti-tumour drug loaded CNT@MS nanocomposites capped with human serum albumin as biocompatible coating were evaluated in vitro with murine breast cancer cells for photothermia
and drug release applications. In this work, such CNTs@Ms were incorporated by blending process in an extracellular-matrix based protein hydrogel (matrigel) to demonstrate their interest for designing smart responsive activable implants. The potential of such nanocomposites as components of smart activable nanocomposite hydrogels to administer antitumor drug release in contact to cancer cells upon NIR light application was shown.

Illustration of the formation of the mixed nanocomposite hydrogels and coating of D2A1 breast cancer cells. Mat. Today Chem. 2020, 17, 100308.
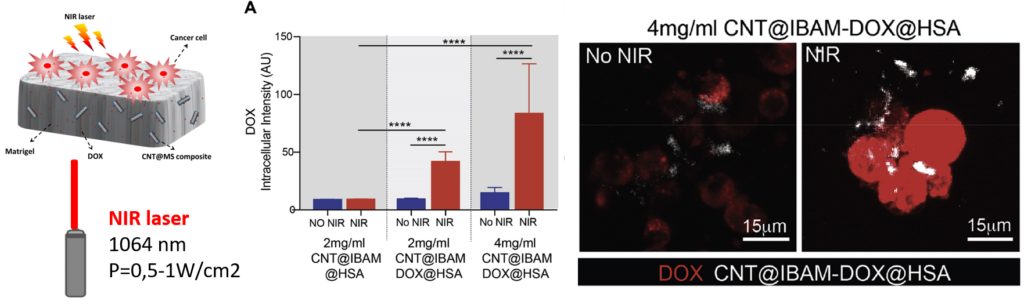
Intracellular red fluorescence intensity of released DOX and confocal microscopy visualization within D2A1 cells grown on hydrogelembedded CNT@IBAM-DOX@HSA without and with NIR laser irradiation. Mat. Today Chem. 2020, 17, 100308.
– Protein nanoreservoirs in composite hydrogels: from burst release to retention
In the area of controlled drug delivery systems, one major issue with hydrogels (HGs) loaded with active
therapeutics such as proteins is their spontaneous leaking from the gel occurring rapidly, usually over several hours. In this work, we address this challenge by demonstrating that the spontaneous protein release from an agarose HG (used as model HG) can be partially or totally blocked by the incorporation of stellate mesoporous silica nanoparticles (STMS, ca. 150 nm size, 15 nm pore) within the HG. We show here that the porous silica NPs act as sub-micron size reservoirs ensuring precise levels retention simply by playing on the amount of STMS embedded in the HG. Further, this effect is shown for various proteins
demonstrating the versatility of this concept.
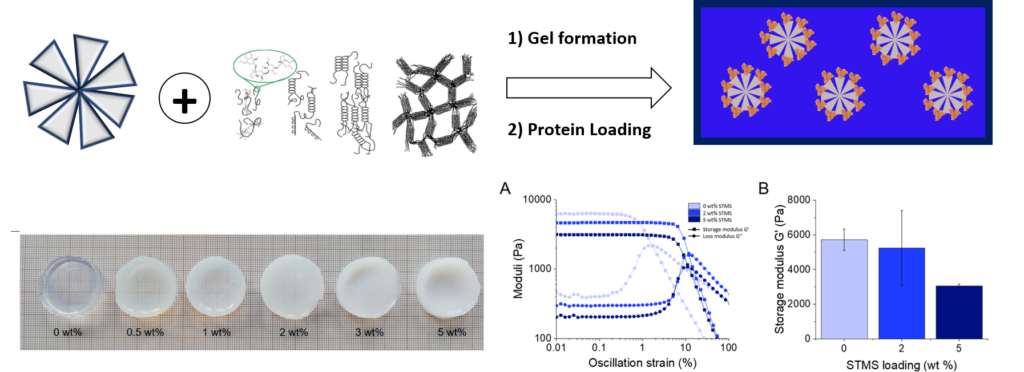
Schematic of the preparation and photos of agarose/STMS composite HG. Mechanical characterization: strain sweeps of the elastic and loss moduli by rheology. Macromol. Chem. Phys. 2024, 225, 2400035.
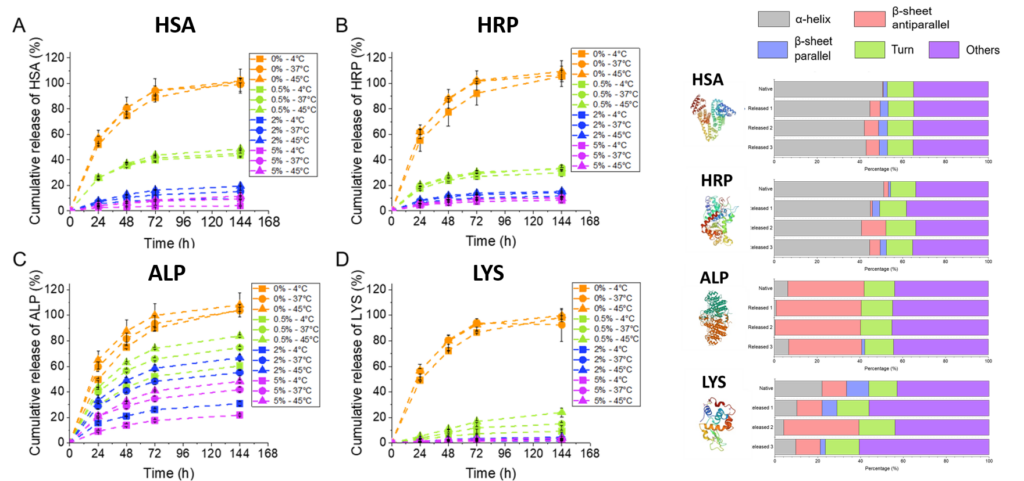
Release profiles at three temperatures of A) HSA; B) HRP, C) ALP and D) LYS when released from an agarose/STMS composite HGs. Secondary structure content obtained from Circular Dichroism spectra of these native and released proteins. Macromol. Chem. Phys. 2024, 225, 2400035.
6-2 Architectured and activable nanocomposite hydrogels
The design of smart nanocomposite supramolecular scaffolds for tissue engineering or anticancer
applications, able to release drugs under external fields is currently a challenge. Such architectures require
not only strong interactions between the polymer matrix and externally responsive nanomaterials, but also an efficient strategy for the loading and release of drugs. Recently, a new biocompatible and effective approach based on the concept of enzyme assisted self-assembly was developed at Institut Charles Sadron, Strasbourg where an enzyme immobilized on on a surface transforms the precursor peptides into hydrogelators.
In this work, in coll with Prof. L. Jierry (ICS, Strasbourg) we addressed an efficient strategy to induce the hydrogelation process from large pore stellate mesoporous silica (STMS). Such STMS NPs, loaded with enzymes, play the role of initiators of the self-assembly process and of cross-linking points for building efficiently the nanocomposite supramolecular hydrogel. Alkaline Phosphatase (AP) and the precursor peptide Fmoc-FFpY (Fmoc: fluorenylmethyloxycarbonyl; F: phenylalanine; Y: tyrosine; p: phosphate group) are used as model systems. An original strategy to immobilize AP on STMS is the use of isobutyramide (IBAM) grafts which allows to immobilize efficiently high amount of AP (100 wt% payload) which there after ensures rapid self-assembly process of peptide (1 hour). Furthermore, an antitumor drug model, doxorubicin (DOX) was successfully loaded along with the formation of homogenously distributed drug-loaded nanocomposite hydrogels (NC HGs). Mechanical reinforcements of the self-assembled peptide hydrogel induced by the AP-immobilized STMS, and also by the loaded drug itself, are shown in a rheology study. At last, the thermo-responsive behavior of such new designed NC HGs was demonstrated highlighting the potential of thermal release of DOX from such architectures.
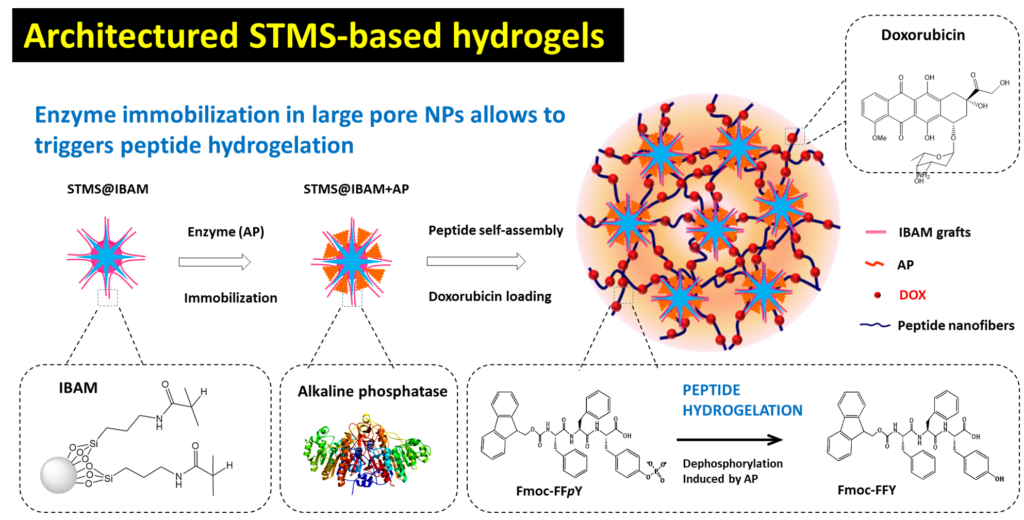
Illustration of the strategy adopted for the chemical design of drug loaded and architectured nanocomposite hydrogels by generating locally at the surface of the nanoparticle self-assembled pepide fibrils hydrogels from enzyme immobilized mesporous silica. ACS Appl. Nano Mater. 2022, 5, 1, 120–125.
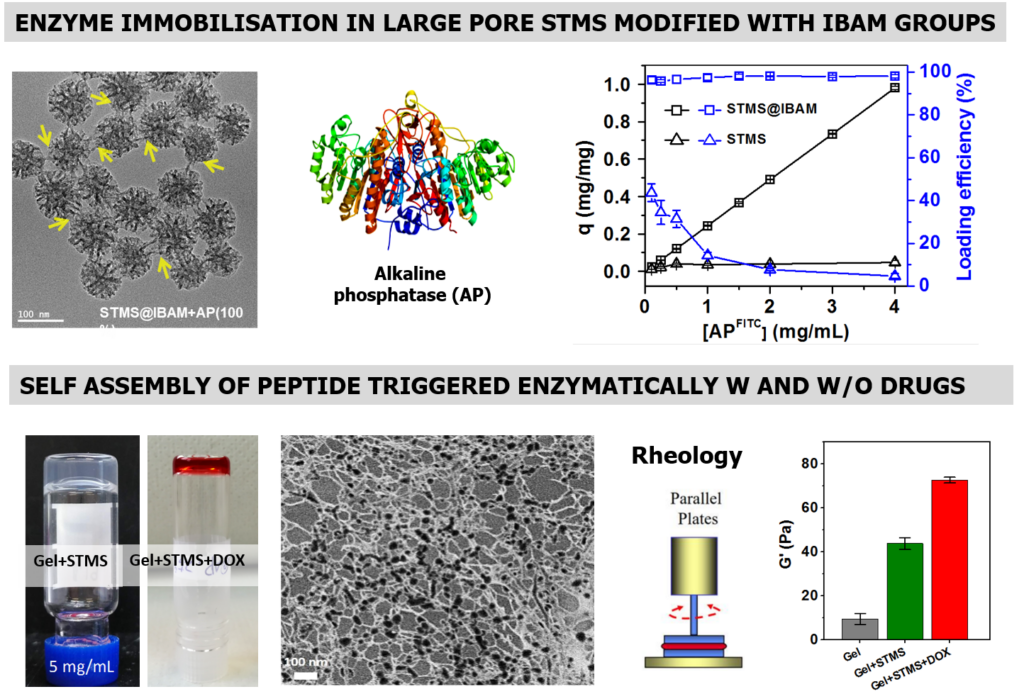
TEM image of AP adsorbed on STMS NPs and AP loading profiles on STMS. Bottom: Inverted tube tests of the self-assembled hydrogels without DOX (left) and with DOX (right). Typical Cryo-SEM images taken from Gel+STMS. Storage modulus (G’) at 1 Hz, and 1% strain of bare hydrogel (Gel), Gel+STMS, Gel+STMS+DOX samples. ACS Appl. Nano Mater. 2022, 5, 1, 120–125.
For the next study, we were interested in triggering the heat release remotely by replacing STMS by an externally activable porous nanomaterial. Among the possible nanomaterials choices, carbon nanotubes (CNTs) are particularly attractive to design nanocomposite scaffolds because they bring high mechanical properties, high electrical conductivity and NIR light induced thermal activation. However, for biomedical applications, given their intrinsic surface hydrophobicity, the capping of such carbon materials with hydrophilic coating is necessary. Among the various capping possibilities the design of large pore silica around CNTs (or other nanomaterial) is very few reported. Hence, CNTs, were coated with large pore (>10 nm) mesoporous silica (CNT@LPMS) which are very suitable for a high loading of enzymes. Then, based on an IBAM-mediated coating, a huge amount of alkaline phosphatase (AP) (>100% wt) was immobilized within the large pores allowing the localized growth of peptide nanofibrous network resulting in supramolecular hydrogel. The incorporation of Doxorubicin (DOX) during the hydrogelation process leads to a reservoir material of anti-cancer agents whose release is photothermally triggered by the NIR light-induced hyperthermia temperature. Combination of rheological studies and molecular simulations indicate an original mechanism in which DOX is included within the peptide nanofibers.
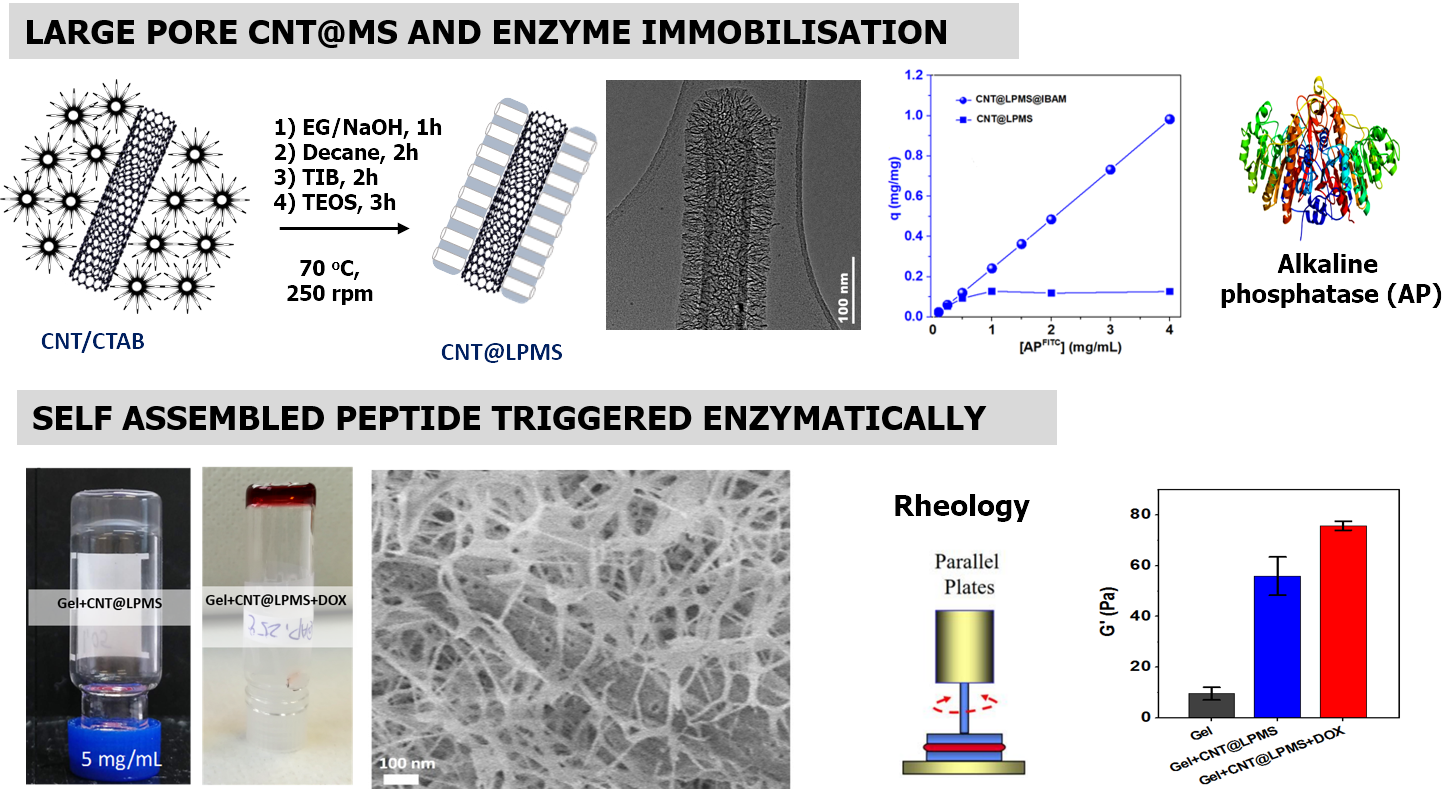
Top : principle to form large pore around CNTs and APs adsorptions profiles on CNT@LPMS. Below : Inverted tube tests of self-assembled hydrogels from AP-immobilized composites: without and with DOX (red gels). Typical Cryo-SEM images taken from supramolecular hydrogel prepared from CNT@LPMS@IBAM+AP (100 wt%) and rheology study. Materialia 2022, 101414.
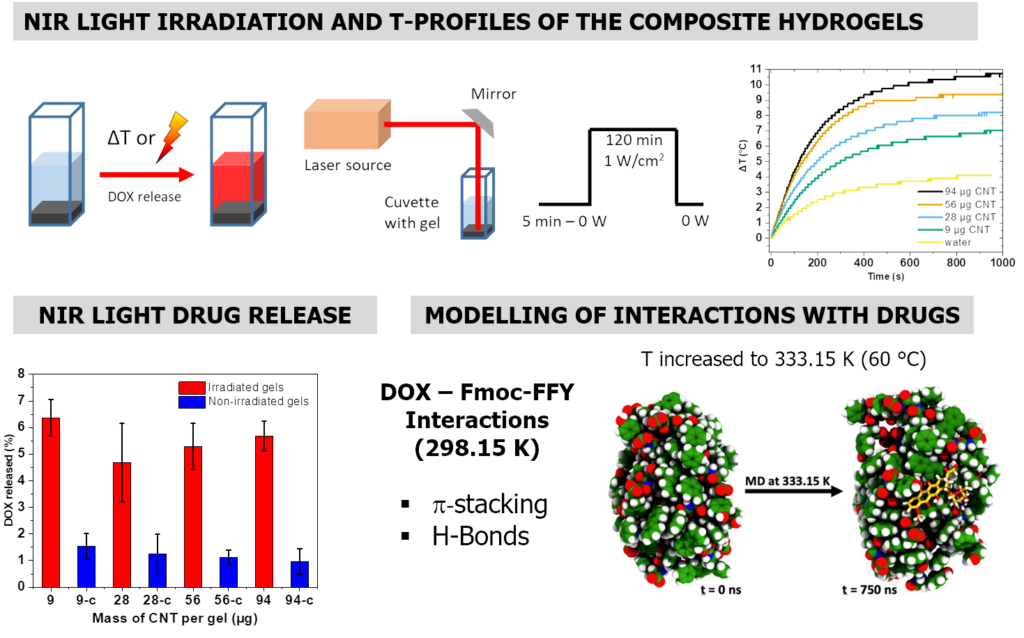
Scheme of the setup to ensure NIR light irradiation and principle of the NIR light-induced drug release. Temperature elevation profiles as a function of the mass of CNT@LPMS composites. DOX released (% vs initial loading) upon NIR light irradiation for 2 h at 1W/cm2. Modelling of interactions between peptides and drugs (coll. A. Chaumont, CMC Strasbourg). Materialia 2022, 101414.
